Ubat, hlm berbaring ing a penting peranan dalam kontemporari penjagaan kesihatan industri, permintaan penyimpanan yang betul untuk mengekalkan keberkesanannya . Soalan biasa ialah: berapa lama boleh kapsul dan tablet lepas habis sebelah mereka pek lepuh s ? Perubatan b pek lister (juga dikenali sebagai pek gelembung) direka bentuk untuk melindungi pil dan kapsul daripada kelembapan, cahaya dan udara, tetapi apabila dibuka, ketahanannya menjadi tidak menentu. Artikel ini mengkaji jangka hayat ubat, kelebihan pembungkusan pek lepuh dan cara memaksimumkan yang kebolehgunaan daripada gelembung bungkus untuk ubat-ubatan sambil memastikan keselamatan produk.
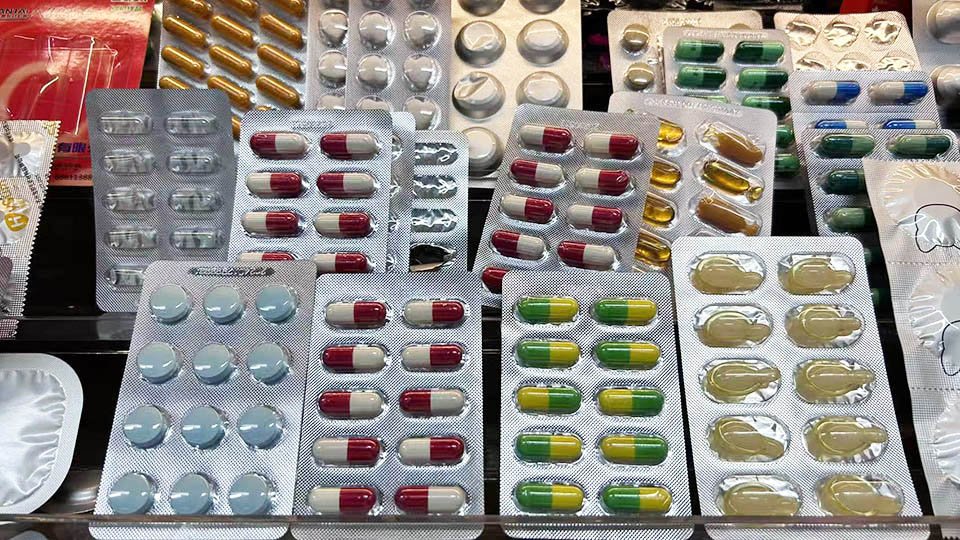 pek lepuh untuk ubat-ubatan
pek lepuh untuk ubat-ubatan
Jangka hayat pil dan kapsul merujuk kepada tempoh di mana ia kekal selamat dan berkesan. Beberapa faktor mempengaruhi tempoh ini, termasuk komposisi kimia, pembungkusan dan keadaan penyimpanan ubat-ubatan pek gelembung. Pengilang biasanya memberikan tarikh tamat tempoh, menunjukkan tempoh masa di mana ubat pembungkusan lepuh mengekalkan s itu potensi—dengan mengandaikan ubat pek lepuh kekal dimeterai dalam asalnya pembungkusan. Walau bagaimanapun, keadaan dunia sebenar boleh mengubah jangka masa ini dengan ketara.
Penyelidikan menunjukkan bahawa pendedahan alam sekitar, terutamanya kepada udara, kelembapan, dan cahaya, adalah faktor utama yang mempengaruhi kestabilan ubat pek lepuh. Setelah dikeluarkan daripada pek lepuh ubat asalnya, tablet ubat menjadi lebih mudah terdedah kepada perubahan fizikal dan kimia. Kelembapan yang tinggi atau turun naik suhu boleh mempengaruhi keberkesanan API . Oleh itu, penyimpanan yang betul dan pembungkusan pelindung adalah pertimbangan utama apabila menentukan berapa lama tablet ubat bertahan di luar perubatan pek lepuh.
|
Ubat Borang |
Kestabilan |
|
Tablet bersalut gula |
Pada mulanya tahan lembapan, tetapi degradasi dipercepatkan jika salutan rosak. |
|
Tablet bersalut filem |
Lebih tahan lembapan dan oksigen daripada tablet tidak bersalut, walaupun pendedahan berpanjangan masih boleh menjejaskan kestabilan. |
|
Kapsul |
Cangkerang gelatin sangat sensitif terhadap kelembapan, yang membawa kepada pelembutan atau pengerasan dalam keadaan buruk. |
|
serbuk |
Sangat sensitif terhadap kelembapan, dengan kecenderungan untuk bergumpal atau kehilangan potensi apabila terdedah. |
Selain itu, ubat pek lepuh seperti tablet bersalut gula, tablet bersalut filem, kapsul dan serbuk mempamerkan tahap kestabilan yang berbeza-beza, dengan setiap jenis menawarkan kelembapan dan perlindungan alam sekitar yang berbeza. Ini menekankan kepentingan lepuh bungkus pembungkusan, yang berfungsi sebagai penghalang berkesan yang disesuaikan dengan berbeza ubat pek gelembung untuk mengekalkan keberkesanannya.
Pek lepuh memainkan peranan penting dalam mengekalkan kestabilan dan keselamatan pil dan kapsul. Direka dengan kerajang pelindung, pek lepuh ubat boleh melindungi setiap dos daripada unsur-unsur yang merosakkan seperti haba, kelembapan, cahaya dan pendedahan oksigen. Penghalang foil amat berkesan dalam menyekat lembapan—salah satu punca utama kemerosotan tablet. Dengan menyegel tablet dalam persekitaran terpencil, pembungkusan lepuh memanjangkan jangka hayat pil dan kapsul, dan memastikan ubat pek gelembung mengekalkan potensinya sehingga tarikh luput.
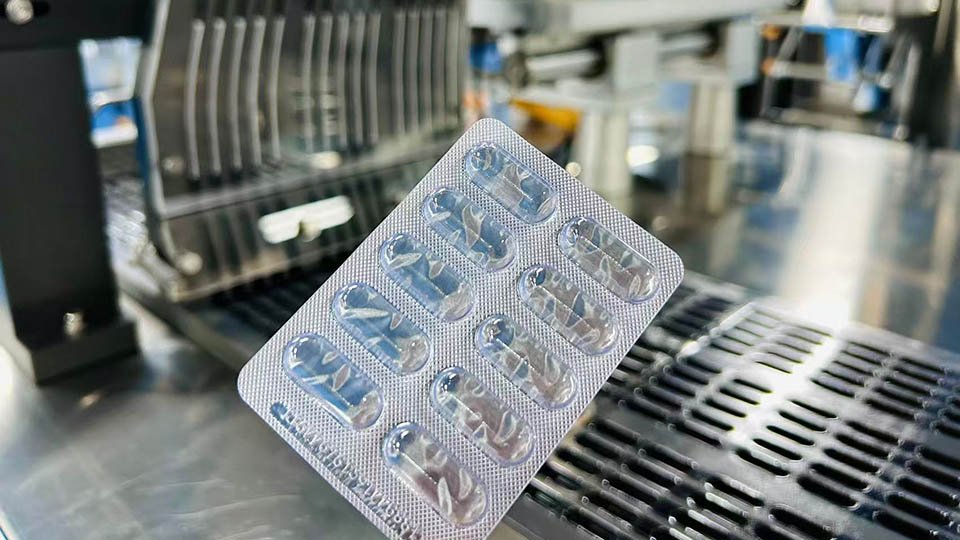 pek lepuh (pek gelembung)
pek lepuh (pek gelembung)
Di luar kemudahan semata-mata, pek ubat gelembung adalah penting untuk mengekalkan kualiti farmaseutikal. Tidak seperti bekas pukal, yang boleh mendedahkan ubat kepada pencemaran, yang ubat blister pack mencegah s hubungan silang dan meningkatkan keselamatan kanak-kanak. Pembungkusan individu ini amat kritikal untuk tablet ubat yang sensitif terhadap kelembapan. Mengeluarkan tablet ubat lebih awal daripada pek gelembungnya boleh mempercepatkan degradasi, menjejaskan keberkesanan tablet.
Bagaimana pula dengan ubat tanpa perlindungan pek lepuh? Apabila ubat pembungkus gelembung dialihkan daripada pek ubat gelembung mereka, faktor persekitaran seperti kelembapan, suhu yang melampau dan cahaya boleh menjejaskan kestabilan mereka dengan ketara. Kelembapan amat berbahaya, kerana ia membolehkan penyerapan air, yang membawa kepada degradasi kimia. Contohnya, tablet yang disimpan dalam keadaan lembap mungkin menghasilkan sisa serbuk, perubahan warna atau struktur yang lemah akibat pendedahan kelembapan dan haba.
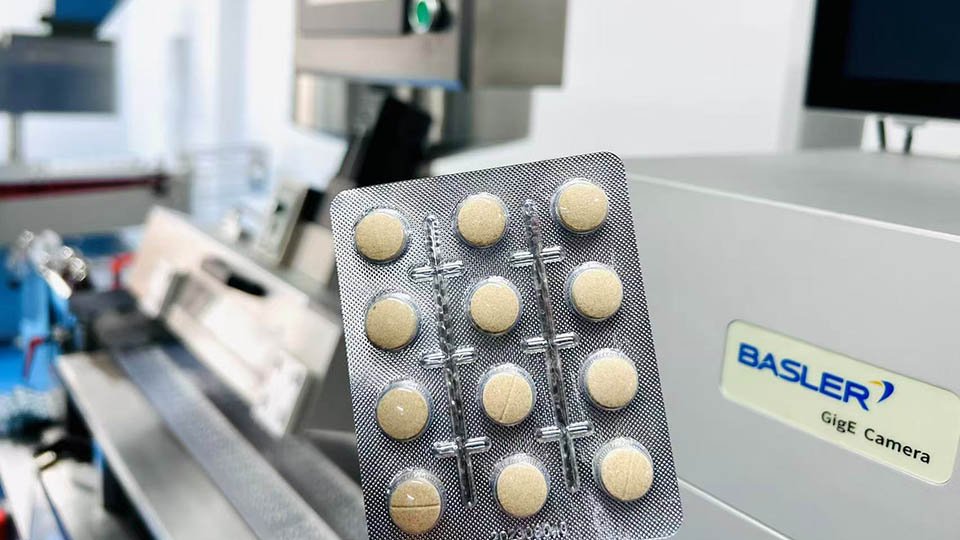 pek lepuh tablet (pek gelembung untuk tablet)
pek lepuh tablet (pek gelembung untuk tablet)
Turun naik suhu juga menimbulkan risiko yang serius. Haba yang berpanjangan mempercepatkan penguraian sebatian aktif, manakala pengembangan dan penguncupan berulang daripada perubahan suhu boleh menyebabkan keretakan atau kerapuhan kepada tablet ubat . Selain itu, cahaya matahari langsung boleh mencetuskan fotodegradasi, lebih rendah ing keberkesanan dru gs . Oleh kerana tablet di luar pek lepuh tidak mempunyai halangan pelindung, penyimpanan yang betul menjadi penting untuk dikekalkan integriti dan keberkesanan mereka.
Pek ubat gelembung biasanya mengandungi maklumat tarikh luput. Tarikh luput berlabel ubat menunjukkan berapa lama tablet ubat kekal selamat dan berkesan di bawah keadaan penyimpanan yang ideal. Kajian mencadangkan bahawa banyak macam-macam ubat bubble wrap mengekalkan sehingga 70% daripada potensinya walaupun selepas tarikh ini jika disimpan dengan betul. Ini selalunya membawa kepada ketidakpastian sama ada pil dan kapsul yang telah tamat tempoh masih boleh digunakan.
Walau bagaimanapun, sebaik sahaja ubat pek lepuh dikeluarkan daripada pek lepuhnya, kestabilannya menurun dengan cepat disebabkan oleh pendedahan kepada udara, kelembapan dan turun naik suhu. Walaupun tablet kelihatan tidak berubah, keberkesanannya mungkin berkurangan dengan ketara. Untuk memastikan keselamatan, pesakit hendaklah sentiasa mematuhi tarikh luput dan berunding dengan ahli farmasi sebelum menggunakan tablet ubat yang disimpan di luar pembungkusan pek lepuh asal mereka.
Pek lepuh pembungkusan adalah digunakan secara meluas dalam industri farmaseutikal, barangan pengguna, dan elektronik kerana itu ketahanan, rintangan gangguan dan keterlihatan. Pengeluaran pek lepuh untuk ubat-ubatan melibatkan satu siri langkah tepat yang dilakukan oleh a mesin pembungkusan lepuh.
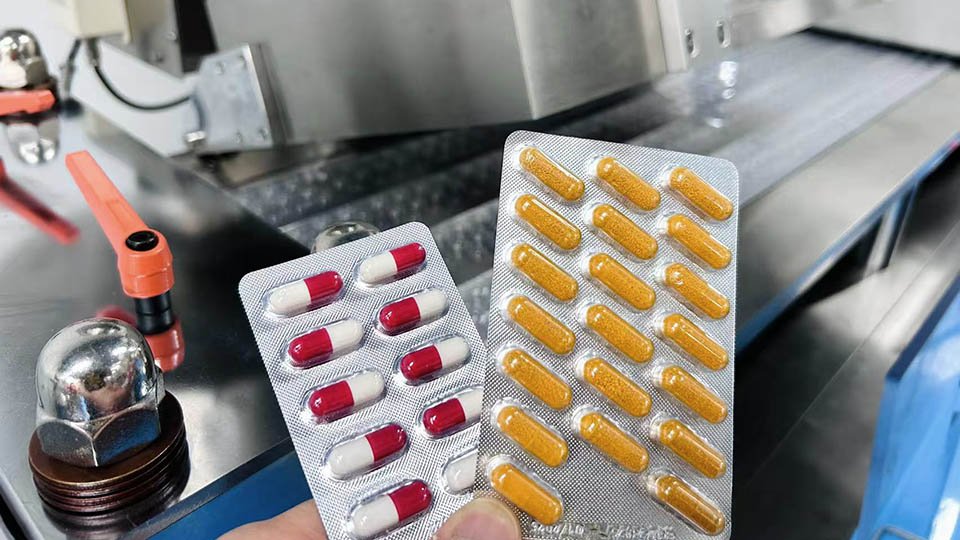 pek lepuh kapsul (pek gelembung untuk kapsul)
pek lepuh kapsul (pek gelembung untuk kapsul)
The pembungkusan lepuh pil proses bermula dengan segulung filem plastik termoform, biasanya diperbuat daripada PVC, PET atau PP. Filem itu akan menjadi disuap ke a lepuh pembungkus dan diproses di bawah tinggi dia di . Stesen pembentuk kemudian menggunakan sama ada vakum, tekanan atau bantuan palam mekanikal untuk membentuk filem yang dilembutkan lepuh rongga yang sesuai dengan bentuk s daripada pil dan kapsul . Kedalaman dan saiz setiap lepuh untuk ubat ialah dikawal dengan tepat kepada memastikan kesesuaian produk yang betul.
Setelah terbentuk, lepuh plastik disejukkan untuk mengekalkan bentuknya s . Lebihan ive filem boleh dipangkas pada peringkat ini untuk memastikan keseragaman sebelum meneruskan ke seterusnya peringkat pemprosesan .
F lembaran lepuh ormed s berpindah ke stesen pengisian, di mana produk, seperti tablet, kapsul, gula-gula, e-rokok, dan picagari kecil, adalah automatiksemua y diletakkan ke dalam setiap lepuh rongga. A high-end lepuh tablet penggunaan mesin s robotik lengan atau penyuap getaran untuk kelajuan tinggi, tepat produk penempatan.
Bahan penutup , selalunya aluminium foil , diletakkan di atas rongga lepuh diisi dengan pil dan kapsul, tablet ubat atau ubat bungkus gelembung lain . Tekanan dan h makan adalah dimanfaatkan kepada dapatkan setiap lepuh bungkus ' s penutup dimeteraikan , memastikan gangguan tahan dan penutupan kedap udara. Beberapa pembungkusan lepuh pil mesin menggabungkan tebuk untuk membuka mudah.
S dimakan ubat lepuh bungkus melalui stesen pemotongan di mana individu gelembung pil pac k ialah dipisahkan menggunakan mekanisme pemotongan mati atau tebukan . F inished ubat buih pek kemudian diperiksa untuk kecacatan sebelum dilepaskan kadbod lepuh selanjutnya pembungkusan.
a sistem penglihatan utomated daripada mesin pek lepuh semak s untuk pengedap yang betul, produk isi ing ketepatan, dan cetakan ing penjajaran (jika penjenamaan digunakan). Pek lepuh yang diluluskan untuk ubat-ubatan dikira dan dibungkus ke dalam karton untuk pembungkusan karton .
Tablet b mesin pembungkusan lister memperkemas proses pakej melalui thermoforming, pengisian tepat, pengedap, dan tepat memotong. Proses automatik ini memastikan kecekapan, perlindungan produk dan pematuhan piawaian industri, menjadikan pek lepuh sebagai penyelesaian pembungkusan pilihan.
Memilih yang boleh dipercayai tablet lepuh bungkus pembungkusan eh adalah penting untuk kecekapan dan keselamatan produk. Model terkemuka , seperti TLT 1400 S Bosch dan RQ Rich Packing DPP 270Maks , menonjol untuk prestasi maju mereka.
Ambil DPP 270Maks mesin lepuh sebagai contoh. Mesin lepuh empat stesen yang dipacu servo sepenuhnya ini pembungkusan ciri berkelajuan tinggi dan automatik lepuh membentuk, produk pengisian, pengedap, ikan kod kelompok e lekukan, potong ing, dan mengira. Mesin pek lepuh ini menawarkan ketepatan yang unggul, operasi yang stabil dan pelarasan pantas. Dikawal oleh sistem PLC, pembungkus pek lepuh pil mengendalikan pelbagai produk , termasuk tablet, kapsul, gula-gula, e-rokok, picagari pakai buang, pil besar dan vial , sesuai untuk pembungkusan aluminium-plastik, kertas-plastik dan aluminium-aluminium. Kepelbagaian dan automasinya membuat mesin melepuh ini pilihan utama untuk industri farmaseutikal dan barangan pengguna.
 mesin pembungkusan lepuh DPP 270Max
mesin pembungkusan lepuh DPP 270Max
Apabila tablet dikeluarkan dari pek lepuhnya, jangka hayatnya berkurangan dengan ketara disebabkan pendedahan kepada kelembapan, udara dan cahaya.
B ubble bungkus untuk ubat-ubatan menyediakan meterai hermetik, melindungi tablet , kapsul keras dan gel lembut daripada degradasi. Setelah dibuka, pil s dan kapsul mungkin bertahan hanya beberapa hari hingga minggu . Isunya — H ow lama pil bertahan — bergantung s pada keadaan persekitaran.
Ubat sensitif kelembapan tablet boleh merosot dengan cepat, kehilangan potensi atau menjadi tidak selamat. Ubat sensitif cahaya tablet boleh rosak lebih cepat w tanpa perlindungan pek gelembung .
Selepas dikeluarkan daripada kepingan lepuh, i f ubat adalah disimpan dalam bekas yang kering dan kedap udara jauh dari cahaya, sesetengah tablet mungkin kekal stabil untuk 1 kepada 3 bulan, tetapi ini jangka hayat berbeza mengikut dadah pelbagai .
Untuk keselamatan, sentiasa semak tarikh luput dan berunding dengan ahli farmasi sebelum menggunakan tablet yang tidak dibungkus. Perubatan b pek senarai adalah penting untuk memelihara integriti dadah. Jangan sekali-kali simpan tablet yang longgar untuk a panjang istilah .
Dari segi jangka hayat farmaseutikal, perbezaan wujud antara ubat preskripsi dan ubat tanpa preskripsi: